Scintillating crystals: a new window in medical imaging
For science fiction fans antimatter is the high energy fuel that accelerates spaceships faster than the speed of light. In the real world however it has more down to Earth yet extremely useful applications, mainly in the medical field. Positrons are used in Positron Emission Tomography (PET) in order to produce three dimensional images of functional processes in the body. How does it work? A sugar, usually fluorodeoxyglucose (FDG), containing a short lived radioactive tracer isotope is injected into the patient. Healthy cells metabolize it at a lesser rate compared to cancerous cells absorb it quickly, as they are more active. The isotope decays, emitting positrons that interact with electrons in the surrounding atoms and annihilate. The annihilation process produces a pair of photons (gamma rays), which move in opposite directions. The scintillating crystals in the PET device detect these photons and the collected data are used to reconstruct an image that reveals the detailed distribution of the isotope and depicts the activity of the cells. More complex metabolic functions can be imaged by using radiolabeled molecules involved in these functions, such as dopamine receptor uptake for the study of schizophrenia or drug addiction.
The interest of CERN in medical imaging dates back to the first activities of Dave Townsend on PET in the 70s. A new phase in this history started with the construction of the LHC and the design of detectors for the four LHC experiments. Paul Lecoq came up with a new initiative; The Crystal Clear Collaboration (CCC) was formed in 1990 and made an extensive investigation for the most adequate scintillator crystals for the LHC. Lead tungstate was chosen by CMS and ALICE as the most cost effective crystal compliant to LHC conditions and today 75848 PWO crystals are installed in CMS and 17920 in ALICE. Crystals developed by the CCC were later used in PET devices. CCC remains active in academic and applied researches on scintillating materials and novel ionizing radiation detecting devices for particle physics and medical imaging, as well as on the development of medical imaging prototypes. The Crystal Clear collaboration is now led by Etiennette Auffray acting as a spokesperson.
ClearPET (a small animal PET scanner)
 A first project to combine nuclear physics and medical imaging, ClearPET, was launched in 1995. It is a small animal PET scanner based on crystals and new photodetector technologies that had been previously developed for HEP experiments. ClearPET is a small animal PET scanner for drug discovery. These scanners are designed for spatial and time resolution imaging needed for a wide range of biomedical and pharmaceutical research applications. ClearPET aimed to apply the non-invasive PET technique to in vivo imaging of animals. The choice of a small animal PET was motivated by the lighter protocol and ethical constraints compared to the case of clinical machines.
A first project to combine nuclear physics and medical imaging, ClearPET, was launched in 1995. It is a small animal PET scanner based on crystals and new photodetector technologies that had been previously developed for HEP experiments. ClearPET is a small animal PET scanner for drug discovery. These scanners are designed for spatial and time resolution imaging needed for a wide range of biomedical and pharmaceutical research applications. ClearPET aimed to apply the non-invasive PET technique to in vivo imaging of animals. The choice of a small animal PET was motivated by the lighter protocol and ethical constraints compared to the case of clinical machines.
Different types of scintillating material were studied and finally a dual layer of phosphor sandwich or phoswich matrix made of LYSO (lutetium yttrium oxyorthosilicate) and LuYAP (lutetium yttrium aluminum perovskite) crystals was used. Thanks to these developments, ClearPET could achieve high localization and very precise measurement with much higher resolution. A second innovation of ClearPET was related to the fact that its diameter was easily adjusted and it could rotate 360º, allowing to perform whole body studies of mice and rats using the small diameter, as well as brain studies of large primates using the large diameter. In other words, the ClearPET System allows measuring all small animals with only one scanner. The project received financial support from CERN as well as from other members of the CRYSTAL CLEAR collaboration and was very successful. It is now licensed and commercialized by the German company Raytest.
ClearPEM
After this success, CCC launched a challenging project, Positron Emission Mammography (ClearPEM), and started looking for new collaborators in 2000. A Portuguese consortium, led by LIP, developed a new system in collaboration with CERN and laboratories participating in the CCC. They aimed to detect cancer cells by focusing on metabolic rather than anatomic differences between healthy and cancerous tissues.
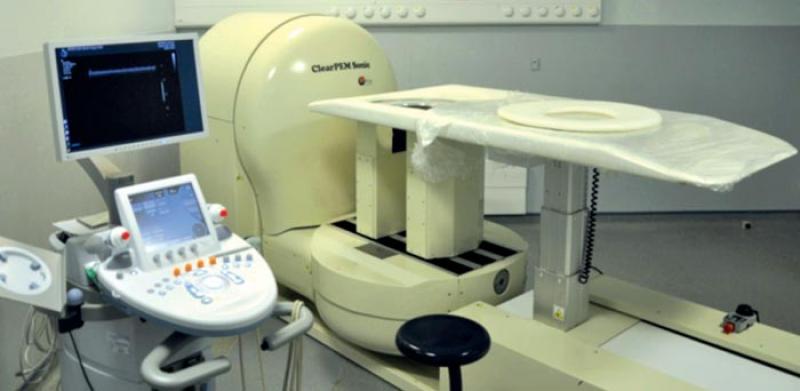
ClearPEM-Sonic installed at Hôpital Nord, in Marseilles. (Image: Benjamin Frisch/CERN)
ClearPEM focused on breast imaging, which lends itself well to this technique. Moreover, breast cancer is the most frequent type of cancer among women and, early detection considerably improves the prognosis for a high survival rate. In about 30% of the cases, standard X-ray mammography doesn’t provide determinant results due to the too high density of the breast tissue, particularly in the cases of young women with dense mammary tissue or women in treatment for menopause. This leads to a large number of unnecessary biopsies that the metabolic approach employed in ClearPEM can significantly reduce. It introduced many technological innovations; each detector plate contains 96 detector matrices and one matrix consists of an 8x4 array of LYSO:Ce crystals, it has 12.000 avalance photodiodes (APD), the largest amount ever used in a medical facility, that was made possible following the large effort placed by the CMS collaboration in the development of APDs. As a result, ClearPEM can identify tumors with a diameter of 2 mm compared to 6-8 mm for a whole body PET. The use of avalance photodiodes along with the use of very compact, very-low background electronics allowed to build a very compact device. Moreover, the configuration of ClearPEM allows the measurement of the depth of interaction (DOI) in the crystals and reduces the parallax error in the lines of response, contributing to better spatial resolution in the reconstructed image. Finally the high sensitivity of the ClearPEM allows a short examination time: about 5mn per breast.
Two ClearPEM prototypes have been built with the help and funding from other partners. The first was installed in Portugal, where the first clinical validation tests have been performed in Porto and in Coimbra. The second one has been installed in Hopital Nord in Marseille and is now moving to the San Gerardo hospital in Monza.
Clinical tests of ClearPEM have been carried out in Marseille on patients with a known breast lesion, who have been injected with FDG for a whole-body PET/CT as part of their diagnostic process. Then the results are compared to conventional imaging and MRI techniques, which all patients participating in the trial have undergone. The ClearPEM image is acquired immediately after the whole-body PET/CT in order to avoid the need for a second injection of FDG to the patient, while the golden standard used for assessment is the histological assessment of the biopsy.
A second challenge for Clear PEM was to combine the machine with an ultrasound system. The combination of PET with ultrasound elastographic imaging will provide co-registered metabolic, anatomical and structural information, therefore the exact location of potential lesions in the surrounding anatomy will be revealed. PEM will collect data about the metabolism of cells, whereas the ultrasound will provide information about the structure and the stiffness of the tumor based on the well-established relation between stiffness and the velocity of sound. Moreover, the availability of elastographic information can further improve the specificity of the examination by identifying non-cancerous diseases, such as benign inflammatory diseases of the breast,– which exhibit higher uptake of the radioactive tracer, fluorodeoxyglucose (FDG) used in PET imaging but are generally softer than a cancerous tumor.
EndoTOFPET (an application of PET to endoscopy)
Recently, CERN-CMS group came up with a proposal for an innovative project that will allow the application of PET techniques to endoscopy. EndoTOFPET-US combines a miniaturized time-of-flight PET detector head with an ultrasonic endoscope. The aim is to develop new biomarkers for prostatic and pancreatic cancer, one of the worst types of cancer in terms of prognosis.
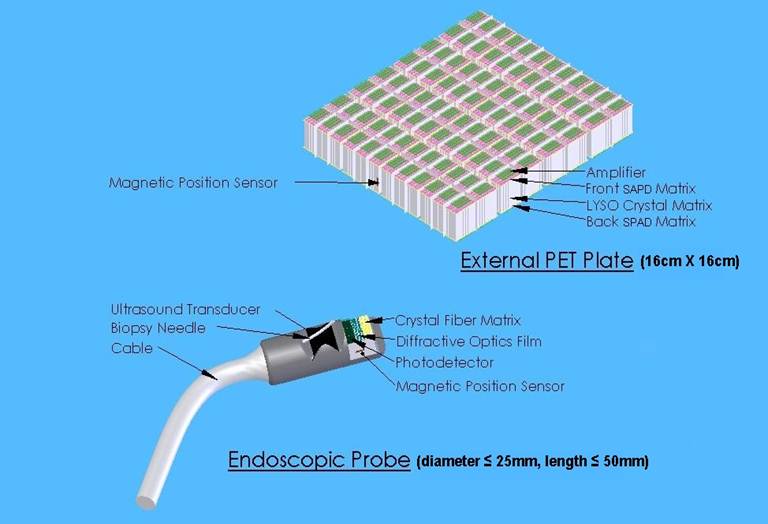
Conceptual design of the endoTOFPET-US Imaging device
EndoTOFPET is largely supported by the 7th European Framework and coordinates a European consortium of six academic institutions, three university hospitals and three companies. It is coordinated by Prof. René Laugier, head of the gastroenterology department of the Timone hospitl in Marseille and by Paul Lecoq from CERN. The main challenge that EndoTOFPET-US has to meet is the non-conventional configuration of organs like the pancreas and the prostate. Endoscopic PET has to manage the high background signals coming from the other organs that surround the pancreas and the prostate, while it has to adjust to the constantly variable geometry-shape of these organs as they are functioning in our body. Moreover, the endoscopic approach, combined with an unprecedented PET time resolution will allow less radiation dose and less invasive imaging, targeting only small internal structures and lesions with the aim of earlier cancer detection and patient-tailored treatment of asymptomatic cancer types.
To reach this goal, great effort went into miniaturizing the crystals and the electronics used in the detector, as well as enhancing light yield. Its design proved to be a challenge as the detector is split in two parts: an external PET plate, consisting of 256 matrices of 4x4 LYSO crystals each coupled to a new generation discrete SiPM matrices with Through VIA technology, and an endoscopic probe consisting of a specifically designed digital SiPM photodetector, a crystal fiber matrix and a diffractive optics film.
Moreover, the internal PET detector requires high granularity, which was achieved by creating a matrix of scintillating crystal fibres with each crystal measuring only 0.75 x 0.75 x 10 mm. The crystals are developed from LYSO and cerium-calcium codoped lutetium oxyorthosilicate (LSO). The internal PET detector is combined with a commercial ultrasound biopsy endoscope adopting different designs for the prostate and the pancreas, as the second presents more stringent anatomical constraints. The external plate has a volume of 20.5 x 20 .x 1.5 cm³ and consists of 4096 LYSO crystals each with 3 x3 x 15 mm³.
One of the novelties is to use fully digital single photo counting approach for the endoscopic probe which should allow ultimate time resolution better than 200 ps, corresponding to a spatial resolution of 3 cm along the Line of Response (LOR). It will also allow faster reconstruction of a 3D image through a limited number of angular projections and efficient rejection of the background outside the region of interest. Paul Lecoq, the initiator of CCC, is very optimistic and believes that this can be achieved by using the very low noise NINO amplifier, with which a coincidence time resolution (CTR) of 188 ps FWHM has been obtained in the configuration of EndoTOFPET.
From a clinical point of view one of the main objectives is to address image-guided diagnosis and minimally invasive surgery with a miniaturized bimodal endoscopic probe with a millimetre spatial resolution and much higher sensitivity than whole-body PET scanners. After a series of preclinical tests on genetically modified pigs to develop prostatic and pancreatic cancers, the team will launch a clinical pilot study on pancreatic and prostate cancer (in a second intention after the present FP7 project), which have almost asymptomatic development and – in the case of pancreatic cancer – extremely bad prognosis if not detected and treated at an early stage.
Based on their experience which has lead to the creation of large European networks, further actions are on the way to federate these actors under a common umbrella and to offer them a translational framework in the form of an open campus (called CERIMED.NET) around CERN, EPFL and the two University Hospitals of Geneva and Lausanne.
Useful Links:
